Multiple Choice
The temperature of an ideal gas in a sealed rigid 0.60- 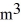
Container is reduced from 460 K to 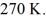
The final pressure of the gas is 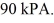
The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K.How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
Definitions:
Related Questions
Q4: If you add 700 kJ of heat
Q16: By what primary heat transfer mechanism does
Q25: A person consumes a snack containing 14
Q46: Your lungs hold 4.2 L of air
Q53: For the graph shown in the figure,what
Q63: Two in-phase loudspeakers are 3.0 m apart.They
Q69: An ideal gas undergoes the process a→b→c→a
Q92: A 50-kg load is suspended from a
Q139: Two pure tones are sounded together and
Q142: An electron,moving south,enters a magnetic field.Because of