The temperature of an ideal gas in a sealed rigid 0.60- 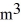
Container is reduced from 460 K to 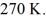
The final pressure of the gas is 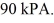
The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K.How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
Definitions:
Q11: A concrete wall of a cold storage
Q28: The resistivity of the material of a
Q40: A small charged plastic ball is vertically
Q48: A sealed container holds 0.020 moles of
Q52: When a 6.00-μF air-filled capacitor has a
Q82: A region of space contains a uniform
Q103: A flat circular wire loop lies in
Q117: When two long parallel wires carry unequal
Q210: The resistivity of gold is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg"
Q217: If emf of the ideal battery is