The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container.The temperature of state 1 is 59°C,the atomic mass of the nitrogen atom is 14 g/mol,and R = 8.31 J/mol ∙ K.What are (a) pressure p1 and (b) temperature T2? 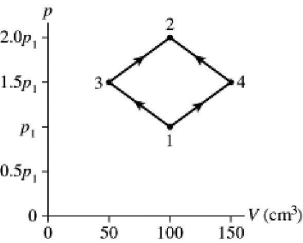
Definitions:
Debt-Equity Ratio
The measure indicating the dual use of debt and equity in the economic structuring of a company’s assets.
Dividend Payout Ratio
The fraction of net income a firm pays to its shareholders in dividends, represented as a percentage.
Profit Margin
A financial metric indicating the percentage of revenue that remains as profit after accounting for costs and expenses.
Retention Ratio
The fraction of net profit not disbursed as dividends, instead held within the company.
Q1: A capacitor C is connected in series
Q11: During an isochoric process,the internal (thermal)energy of
Q26: The figure shows conventional current passing through
Q38: Two processes are shown on the pV
Q56: An electron moving along the +x-axis enters
Q71: The density of water at 0°C is
Q101: Two metal rods are to be used
Q119: What is the intensity of a 70-dB
Q144: For the circuit shown in the figure,V
Q204: In the circuit shown in the figure,the