An expansion process on an ideal diatomic ideal gas has a linear path between the initial and final coordinates on a pV diagram.The coordinates of the initial state are: the pressure is 300 kPa,the volume is 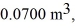
And the temperature is 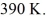
The final pressure is 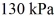
And the final temperature is 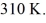
How much work is done by the gas during this process? (R = 8.31 J/mol ∙ K)
Definitions:
Price Floor
A government-imposed minimum price set above the equilibrium price, preventing market prices from falling below it.
Shortage/Surplus
Conditions where the quantity demanded is greater than the quantity supplied (shortage) or the quantity supplied is greater than the quantity demanded (surplus) in a market.
Quantity Demanded
The total amount of goods or services that consumers are willing and able to purchase at a specific price level.
Quantity Supplied
The volume of a good or service available for sale from suppliers at a certain cost.
Q29: Two +6.0-µC charges are placed at two
Q35: For the circuit shown in the figure,C
Q43: A certain ideal gas has a molar
Q47: A fluid in an insulated,flexible bottle is
Q65: An asteroid of mass 58,000 kg carrying
Q77: When current is flowing in an ordinary
Q85: What current flows from the battery in
Q97: A battery is rated such that it
Q140: A train is traveling away from you
Q224: If a quantity you calculated has units