How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 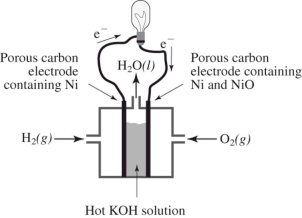
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water.
II.Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen.
III.In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction.
IV.It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell.
Definitions:
Lost Dog
A canine that has gone missing from its owner's home or care.
Groceries
Items of food and other household necessities purchased for consumption and use within the home.
Dickinson V. Dodds
A landmark case in contract law, dealing with the revocation of offers.
Revoked
The act of officially canceling or withdrawing something, such as a license, permission, or legal right.
Q3: Senior officers in a national military organization
Q4: What is meant by a "citizen petition"?<br>A)It
Q5: The most numerous of the elements are
Q34: Which is/are concerns about genetically modified food
Q44: Generally,the water that has the least harmful
Q45: The correct name for CuO is<br>A)copper oxide.<br>B)monocopper
Q46: Which butter substitute has the highest percentage
Q53: The basal metabolism rate (BMR)is<br>A)the minimum amount
Q145: Which source of power tends to produce
Q157: Communications,marketing,and information systems are three emerging fields