How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 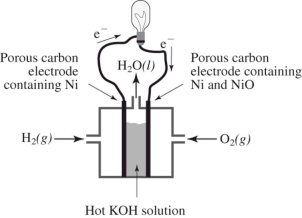
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water.
II.Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen.
III.In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction.
IV.It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell.
Definitions:
New Product
An item or service recently introduced to the market that offers novel features or benefits not previously available.
Value Barrier
Challenges or factors that limit the perceived worth or utility of a product or service to potential customers.
New-Product Adoption
The process by which consumers or businesses start using a new product, potentially going through stages from awareness to acceptance and regular use.
Cereal Bar
A snack or breakfast food made from cereals and various additives, often sweetened and packaged for convenience.
Q4: What distinguishes the atoms of one element
Q13: Sunlight (solar radiation)may be turned directly into
Q17: Which incorrectly pairs over-the-counter drugs with its
Q46: What type of radiation is given off
Q50: The molecule(s)being acted upon by catalysts is(are)<br>A)active
Q52: The general formula for coal is C<sub>135</sub>H<sub>96</sub>NOS,the
Q57: The influence strategy called "exchange" applies:<br>A)negotiation.<br>B)ingratiation.<br>C)persuasion.<br>D)nonsubstitutability.<br>E)both negotiation
Q59: Identify the chiral carbon atom in L-dopa
Q65: Which is an oxidation half-reaction?<br>A)2 H<sub>2</sub> +
Q84: Which organizational behaviour perspective discusses inputs,outputs,and feedback?<br>A)Mechanistic