Multiple Choice
Based on this reaction and its energy profile, 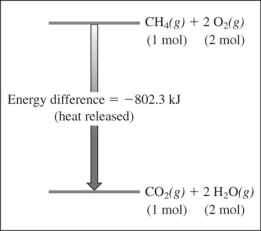
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
The reaction
Definitions:
Related Questions
Q6: The pH of lemon juice is approximately
Q9: Which is not a consequence of hydrogen
Q14: Which statement is not true?<br>A)In forming a
Q15: Which would contribute most to your annual
Q25: Which of the following contains the most
Q35: Which is the best definition of specific
Q63: Which is the cathode in this galvanic
Q65: Calculate the mass of 25,000 molecules of
Q68: Which statement is not true?<br>A)From discovery to
Q75: What are the steps involved in the