For the following question(s) ,consider the following balanced equation. 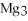
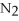 + 6
+ 6 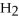 O → 3
O → 3 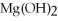 + 2
+ 2 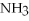
-When 4 moles of aluminum are allowed to react with an excess of chlorine gas, 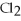 ,how many moles of aluminum chloride are produced?
,how many moles of aluminum chloride are produced?
2Al + 3 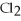 → 2Al
→ 2Al 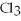
Definitions:
Capacity
The maximum level of output that a company can sustain to make a product or provide a service under normal conditions.
Q22: What is the IUPAC name for a
Q33: When naming an alkene,the parent chain is
Q34: 0.705 m
Q36: Radon is a metal.
Q42: Gas law calculations require the use of
Q71: Which of the following is an example
Q71: 680 000 km
Q77: A sulfur atom gains electrons to form
Q99: According to the atomic theory,_.<br>A)all atoms are
Q122: What type of compound is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg"