For the following question(s) ,consider the following balanced equation. 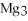
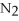 + 6
+ 6 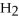 O → 3
O → 3 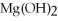 + 2
+ 2 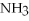
-In the reaction of nitrogen gas, 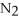 ,with hydrogen gas,
,with hydrogen gas, 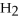 ,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen?
,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen? 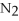 + 3
+ 3 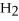 → 2
→ 2 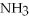
Definitions:
Game Console
A dedicated electronic device designed to play video games through a connection to a television or other display screen.
Highly Concentrated
Refers to a market structure where a small number of firms hold a large portion of the market share.
Natural Monopoly
A market condition where a single firm can provide goods or services to an entire market at a lower cost than two or more companies could, due to high fixed costs and significant economies of scale.
Economies of Scale
The financial benefits gained by businesses as a result of their operating size, where the cost for each unit produced decreases as the scale of operation grows.
Q5: A homogeneous mixture that does not settle
Q18: What is the IUPAC name of <img
Q24: According to the kinetic theory of gases,particles
Q42: In the IUPAC naming system,an aldehyde is
Q60: What is(are)the product(s)of the complete combustion of
Q62: Which of the following gives the correct
Q71: What is the product when this compound
Q81: The dosage of technetium-99m for myocardial imaging
Q88: A patient has a temperature of 38.5
Q94: How many grams of hydrogen are needed