Answer the question(s) below about the following reaction.
2 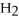
 → 2
→ 2 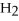 O +
O + 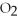
-How many moles of oxygen gas can 5.0 moles of hydrogen peroxide (H2O2) produce,if decomposition is complete?
Definitions:
Application
The act of putting something to a particular use or purpose, or a formal request for something, typically a job or acceptance into an educational institution.
Current Economy
The prevailing conditions of an economic system at a specific time, including factors such as employment rates, GDP, and market trends.
Career Strategies
Plans and techniques employed to navigate and advance one's professional life.
Part-Time
Employment or engagement in activities for only a portion of the usual working hours.
Q2: In any balanced chemical equation,the number of
Q5: How many grams of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="How
Q6: When the measured number 0.0090 is multiplied
Q12: What is the molarity of a solution
Q21: In nuclear fusion,a large atom splits into
Q25: In this reaction,what is the coefficient for
Q37: _ can pass through filters but cannot
Q46: On a heating curve a plateau corresponds
Q61: Using a kidney machine to remove waste
Q62: The formation of a gas resulting from