For the following question(s) ,consider the following balanced equation. 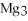
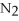 + 6
+ 6 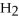 O → 3
O → 3 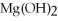 + 2
+ 2 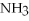
-When 4 moles of aluminum are allowed to react with an excess of chlorine gas, 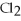 ,how many moles of aluminum chloride are produced?
,how many moles of aluminum chloride are produced?
2Al + 3 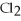 → 2Al
→ 2Al 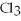
Definitions:
Smoking Cessation
The process of discontinuing tobacco smoking, which can significantly reduce the risks of developing smoking-related diseases.
Health Belief Model
An intellectual model aimed at understanding and anticipating behaviors related to health by scrutinizing individual attitudes and beliefs.
Predictive Value
The extent to which a test can accurately predict the presence or future occurrence of a condition or disease.
Ethnic Groups
Communities or populations sharing common cultural traits, ancestry, language, history, society, and, in most cases, geography.
Q17: One mole of copper(II)sulfate,CuS <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="One
Q17: One atmosphere is the same pressure as
Q21: A car driving on the freeway has
Q26: In the kinetic molecular theory of gas
Q37: -7 °C
Q61: The name of the compound CuSO<sub>4 </sub>is
Q70: The temperature is changed from 50 °C
Q72: Iodine-123,which is used for diagnostic imaging in
Q80: Which one of the following elements forms
Q82: What is the name for a two-carbon