For the following question(s) ,consider the following balanced equation. 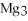
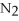 + 6
+ 6 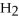 O → 3
O → 3 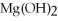 + 2
+ 2 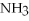
-In the reaction of nitrogen gas, 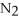 ,with hydrogen gas,
,with hydrogen gas, 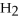 ,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen?
,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen? 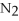 + 3
+ 3 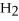 → 2
→ 2 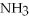
Definitions:
Penal System
The network of laws, institutions, and administrative practices aimed at deterring crime and punishing those who violate laws.
Canadian Prison
A facility in Canada where individuals who have been convicted of crimes are confined as a form of punishment and rehabilitation.
Rehabilitation
Involves curing disabilities to the extent possible through medical and technological intervention; trying to improve the lives of people with disabilities by means of care, training, and education; and integrating people with disabilities into society.
Productive Members
Individuals who contribute positively to society through their work, community service, or by fulfilling societal roles that support economic and social development.
Q1: A patient receives 3.0 mL of a
Q14: 9.00 × <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="9.00 ×
Q25: Hypoglycemia is a condition in which _.<br>A)the
Q42: Gas law calculations require the use of
Q46: At STP conditions,11 g of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg"
Q56: How many electrons will chlorine gain or
Q58: According to the Brønsted-Lowry definition,_.<br>A)an acid is
Q61: How many grams of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6077/.jpg" alt="How
Q74: carbon dioxide
Q82: What is the name for a two-carbon