For the following question(s) ,consider the following balanced equation. 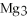
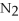 + 6
+ 6 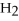 O → 3
O → 3 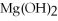 + 2
+ 2 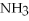
-How many grams of NO are required to produce 145 g of 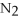 in the following reaction?
in the following reaction?
4 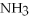 + 6NO → 5
+ 6NO → 5 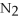 + 6
+ 6 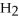 O
O
Definitions:
Streetlight
A light, typically mounted on a pole, providing illumination for a street or public area to enhance safety and visibility at night.
Willing
Generally refers to being prepared or inclined to do something, indicating a readiness or acceptance.
Public Good
Public goods are goods that provide benefits to all members of a society, without the ability to exclude individuals from enjoying the benefits, such as clean air or national defense.
Marginal Benefit Curve
A graphical representation showing how the benefit to consumers changes as the quantity of a good consumed increases.
Q8: Octane is a seven carbon linear alkane.
Q19: Stereoisomers that are nonsuperimposable mirror images of
Q19: The molarity (M)of a solution refers to
Q49: The rem is a unit that measures
Q66: A solution which has 12 g of
Q70: The nuclear reaction shown below is an
Q75: What is the condensed structural formula for
Q76: The number of particles in 1 mole
Q84: Steam at 100 °C contains the same
Q114: An unsaturated compound always _.<br>A)is a cycloalkane<br>B)contains