The reaction for the combustion of heptane is 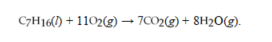 . How many liters of CO2 at STP are produced from the complete combustion of 2.00 moles of heptane?
. How many liters of CO2 at STP are produced from the complete combustion of 2.00 moles of heptane?
Definitions:
Pure Monopolist's
A market structure in which a single firm has exclusive control over the supply of a good or service with no close substitutes, giving it the power to set prices.
Profit-Maximizing
The process or goal of a firm to adjust output and pricing to achieve the highest possible profit.
Marginal Revenue
The increased income derived from selling an additional unit of a product or service.
Pure Monopolist
A single seller in a market with no close substitutes for the product or service, having complete control over its price.
Q7: The number of moles of oxygen gas
Q10: When calcium and oxygen combine,the formula of
Q23: The name of the compound Fe<sub>2</sub>S<sub>3</sub> is
Q54: A gas sample in a closed,expandable container
Q56: What is the molar mass of <img
Q57: Which has a higher osmotic pressure 1.0
Q60: Formaldehyde is used industrially to make _.<br>A)polymers<br>B)insulating
Q62: Amylose is a polysaccharide.
Q92: What kind of taste do carboxylic acids
Q114: An unsaturated compound always _.<br>A)is a cycloalkane<br>B)contains