Given the table of specific heat values below,what is the identity of a 26.2 g metal sample that increases by 8.5°C when 100.0 J of energy is absorbed? 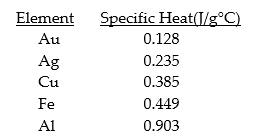
Definitions:
Cash Surrender Value
The amount an insurance policyholder is entitled to receive if they decide to terminate the policy before it matures or an insured event occurs.
Annual Premiums
Annual premiums are the amount paid yearly for insurance coverage or other similar policies.
Insurance Expense
The cost associated with purchasing insurance policies to protect against risks, recognized regularly over the term of the policy.
Outstanding Common Stock
Refers to the shares of a corporation that have been issued and are currently owned by investors, including public shareholders and company insiders, but excluding shares owned by the corporation itself.
Q22: In comparing a balloon containing 25 grams
Q45: What is the mass percent of hydrogen
Q63: The correct number of significant figures in
Q65: When rounding the number 2.348615 to 4
Q76: The nucleus of an atom is a
Q82: At a round-table marketing meeting for a
Q86: What is the volume of 19.6 g
Q97: The chemical formula CH<sub>2</sub>O can be classified
Q127: The distance from New York City to
Q174: An ultimate consumer is considered someone who:<br>A)uses