Diatomic O2 can react with the element magnesium to form magnesium oxide (MgO) .The balanced chemical equation is: 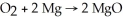
If 4 moles of magnesium totally reacted with more than enough O2,how many moles of MgO would be expected to form?
Definitions:
Non-Controlling Interest
A share of equity ownership in a subsidiary not attributable to the parent company, representing the portion of the subsidiary's net assets and net income not owned by the parent.
Subsidiary
A company that is controlled by another company, known as the parent company, through ownership of more than 50% of its voting stock.
Interest Revenue
Income earned on investments or money lent, represented by the amount charged to borrowers for the use of the lender's funds.
Elimination
In accounting, the process of removing intercompany transactions from the financial statements of a consolidated group.
Q14: When the equation _NO<sub>2</sub> + _H<sub>2</sub>O +
Q20: The Lewis structure of water has two
Q29: Ernest Rutherford proved the existence of electrons.
Q42: What type of reaction is the generic
Q43: The Lewis model predicts that the formula
Q50: What is the limiting reactant for the
Q61: A precipitate is expected to be formed
Q72: Which of the below statements about global
Q81: A 500.gram iron ore sample was determined
Q105: Identify the double displacement reactions among the