A balanced chemical equation used to prepare ammonium carbonate,(NH4) 2CO3 ,is: 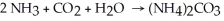
Which choice of reactant quantities shown below would result in the greatest amount of ammonium carbonate being formed?
Definitions:
Triglycerides
A type of fat found in the blood that the body uses for energy.
Low-Density Lipoprotein
Often referred to as "bad" cholesterol, it is a type of lipoprotein that transports cholesterol to cells throughout the body, which can lead to plaque buildup in arteries.
Hypertension
A medical condition in which the blood pressure in the arteries is persistently elevated.
Blood-Alcohol Level
The amount of alcohol present in the bloodstream, usually measured to determine intoxication level.
Q15: The subshells of the orbital are represented
Q17: What is the mass percent of carbon
Q21: Which intermolecular force is due to the
Q47: Which subshell letter corresponds to a 4-leaf
Q65: An iron ore sample is found to
Q77: How many lone electron pairs does the
Q81: Which formula shows the proper use of
Q86: Gas density can be calculated by dividing
Q96: What is the initial temperature of a
Q108: A photon represents the mass of a