Which is the limiting reactant in the following reaction given that you start with 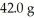 of CO2 and 99.9 g KOH?
of CO2 and 99.9 g KOH?
Reaction: CO2 + 2KOH → K2CO3 + H2O
Definitions:
Future Value
The value of an investment at a specified date in the future that is equivalent in value to a specified sum today, after being compounded at a given interest rate.
Simple Interest
Interest calculated on the principal amount of a loan or deposit, without compounding.
Account Value
The total worth of a financial account, including cash, investment holdings, and any other assets minus liabilities.
Present Values
Present values represent the sum total of all future cash flows of an investment or project discounted back to their value today.
Q9: What is the formula mass of magnesium
Q35: What is the correct Lewis structure for
Q42: Which statement below is false?<br>A)A hydrogen bond
Q48: What is the mass of 1.56 ×
Q49: Which one of the following is the
Q53: The reaction of carbonate ion with magnesium
Q66: The following reaction IS balanced: BaCl<sub>2</sub> +
Q70: The elements with the highest electronegativity values
Q108: What is the oxygen-to-hydrogen mass ratio for
Q115: The solubility of solids in water generally