Diatomic N2 can react with diatomic H2 to form ammonia (NH3) .The balanced chemical equation is: 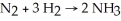
If 6 moles of H2 totally reacted with more than enough N2,how many moles of ammonia would be expected to form?
Definitions:
Deposit Slip
A document that accompanies bank deposits, detailing the amount of cash and/or checks being deposited.
Currency
A system of money in general use in a particular country.
Check Register
A record of all checks written, deposits made, and charges or credits to a bank account.
Cash Balance
The amount of cash in a company's account at any given time.
Q12: What is the pressure of 760 mm
Q44: Consider a reaction of chemicals depicted as
Q63: Given the recipe: 2 cups flour +
Q64: How many atoms are in 1.50 moles
Q72: The distance between adjacent wave crests is
Q86: How many moles of carbon are in
Q96: What are the coefficients for the following
Q98: Which of the following elements has the
Q98: The mass of a proton is exactly
Q109: Which of the following statements about empirical