Diatomic O2 can react with the element magnesium to form magnesium oxide (MgO) .The balanced chemical equation is: 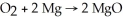
If 4 moles of magnesium totally reacted with more than enough O2,how many moles of MgO would be expected to form?
Definitions:
Meeting of the Minds
An agreement between parties on the terms of a contract, demonstrating mutual consent and understanding.
Unilateral Mistake
An error made by one party in a contract that does not affect the other party's understanding or agreement.
Misunderstanding
A situation where the intended meaning of a communication is not correctly perceived by one or more parties.
Fraudulent Misrepresentation
A false statement made knowingly or recklessly with the intent to deceive, leading to a contract or transaction.
Q6: An atom that has the same number
Q49: Which one of the following is the
Q53: The reaction of carbonate ion with magnesium
Q69: How many atoms are in 15.6 grams
Q75: The unit of pressure known as the
Q80: A pedometer is a device created by
Q82: The energy of each Bohr orbit increases
Q89: Water and oil do not mix because
Q95: Given the recipe: 2 cups flour +
Q111: How many moles of NH<sub>3</sub> can be