Consider the following electronegativity values: 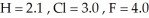 Which molecule below would you expect to have the more polar bond?
Which molecule below would you expect to have the more polar bond?
Definitions:
Sarbanes-Oxley Act
A federal law established in 2002 aimed at improving corporate governance and accountability, in response to financial scandals.
Securities Fraud
A type of serious financial fraud involving the manipulation of the securities markets, such as insider trading or misleading statements by companies.
Q8: Boyle's Law is expressed as:<br>A)V is proportional
Q16: What molarity should the stock solution be
Q19: Solubility of gases in water:<br>A)is independent of
Q43: The Lewis model predicts that the formula
Q52: Which of the following is a weak
Q76: After you have completed the task of
Q96: What is the initial temperature of a
Q104: What type of reaction is the generic
Q107: Having eight valence electrons is very stable
Q110: The molarity of a solution prepared by