Which of the following diatomic elements would have a mass of 19.08 grams stored in a 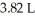 container at
container at 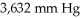 and 100°C? (R= 0.0821 L atm/ mol K)
and 100°C? (R= 0.0821 L atm/ mol K)
Definitions:
Profit Maximizing
The procedure a business uses to ascertain the most profitable price and volume of output.
Monopolist
A single seller in a market, who has significant control over the price and supply of a specific good or service.
Marginal Cost
The increase in total cost resulting from producing one additional unit of a good or service.
Price Discriminate
A pricing strategy where a seller charges different prices for the same product or service to different customers, based not on costs, but on willingness to pay.
Q6: Which of the following is NOT an
Q8: Boron forms compounds that violate the octet
Q9: What are the coefficients for the following
Q10: Without intermolecular forces,solids and liquids would not
Q72: The equilibrium constant, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6110/.jpg" alt="The equilibrium
Q84: If the kelvin temperature of a gas
Q95: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6110/.jpg" alt="For the
Q95: Given the recipe: 2 cups flour +
Q100: Calculate the molarity of a KCl solution
Q108: In order to determine the limiting reactant