Which of the following diatomic elements would have a mass of 19.08 grams stored in a 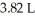 container at
container at 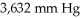 and 100°C? (R= 0.0821 L atm/ mol K)
and 100°C? (R= 0.0821 L atm/ mol K)
Definitions:
Amino Acid
Organic compounds that serve as the building blocks of proteins, containing both amino and carboxylic acid functional groups.
Codon
A sequence of three nucleotides in mRNA that specifies the addition of a specific amino acid or the release of a polypeptide chain during protein synthesis.
Alanine
A non-essential amino acid used in the biosynthesis of proteins.
AAC
May refer to Advanced Audio Coding, a digital audio codec designed for lossy compression of audio data.
Q14: Which molecule listed below is a nonpolar
Q14: One liter of 6.0 M HNO<sub>3</sub> contains
Q27: For an ideal gas,which of the following
Q47: Which subshell letter corresponds to a 4-leaf
Q56: The tendency of a liquid to minimize
Q71: The vapor pressure of water is independent
Q84: If the kelvin temperature of a gas
Q94: We have the following reaction at equilibrium
Q94: Substances that can act both as an
Q101: A rigid cylinder contains 2.00 liters of