What is the change in the freezing point of a solution made by dissolving 14.7g of C6H12O6 into 150.0 ml of water? The density of water is 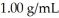 and
and 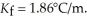
Definitions:
Actors
Individuals who perform in stage plays, movies, television shows, or other productions, portraying characters.
Observers
Individuals who watch or notice something without directly participating in the action.
Private Thoughts
Thoughts that are not expressed verbally or shared with others, often personal or reflective in nature.
Talking
The act of engaging in spoken communication to convey messages, information, or express thoughts and emotions.
Q2: Tap water contains dissolved nitrogen and oxygen.
Q18: Which of the following are typically TRUE
Q49: Which of the following is the active
Q53: How many grams of KCl are needed
Q54: Which molecule listed below is a polar
Q79: In the following reaction: HCO<sub>3</sub><sup>- </sup>(aq)+ H<sub>2</sub>O
Q106: Lewis theory predicts that the formula for
Q109: The larger the equilibrium constant,the greater is
Q114: What is the concentration of hydronium ions
Q118: The initial volume of a gas cylinder