Calculate the equilibrium constant for the reaction below given that 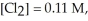
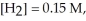 and
and 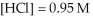 at equilibrium.
at equilibrium.
Given: Cl2(g) + H2(g) ⇌ 2HCl(g)
Definitions:
Generation X
A demographic cohort following the baby boomers, typically defined as people born from the early-to-mid 1960s to early 1980s.
Generation Z
Generation Z refers to the cohort of people born approximately between the mid-1990s to early 2010s, known for their familiarity with the internet, technology, and digital communications from a young age.
Baby Boomers
The demographic group born approximately between 1946 and 1964, known for significant population growth and cultural impact.
Reverse Mentoring
Programs that provide younger workers an ability to mentor and teach older colleagues.
Q3: Canadian stocks are cross-listed in the United
Q8: What is the concentration of H<sup>+</sup> in
Q39: Which substance would be expected to have
Q49: The reaction CH<sub>3</sub>CH<sub>3</sub> + Br<sub>2</sub> → CH<sub>3</sub>CH<sub>2</sub>Br
Q53: A catalyst speeds up a chemical reaction
Q61: A straight chain saturated hydrocarbon has eight
Q83: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6110/.jpg" alt="For the
Q92: The exact shape that a protein takes
Q103: When writing the expression for an equilibrium
Q123: What is the molecular weight of a