Calculate the equilibrium constant for the reaction below given that 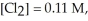
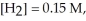 and
and 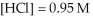 at equilibrium.
at equilibrium.
Given: Cl2(g) + H2(g) ⇌ 2HCl(g)
Definitions:
MSR / MSE
MSR (Mean Square Regression) and MSE (Mean Square Error) are measures in regression analysis, where MSR evaluates the variance explained by the model, and MSE measures the average error of the model’s predictions.
F-test Statistic
A statistical test used to compare the variances of two populations based on sample data from those populations.
Multiple Linear Regression Model
A statistical technique that models the relationship between one dependent variable and two or more independent variables.
Partial Regression Coefficients
Parameters in a multiple regression model that measure the impact of a change in one predictor variable on the response variable, holding all other predictors constant.
Q2: When sufficient quantity of heat has been
Q9: Which compound will have the highest boiling
Q18: Eurodollar interest rate futures contracts have all
Q23: How many liters of a 2.18 M
Q25: What is the main product when <img
Q39: What is the oxidation state of nitrogen
Q47: Which statement below is FALSE?<br>A)The rate of
Q54: The complementary base sequence to GTAGCT is:<br>A)CATCGA.<br>B)ACGATC.<br>C)GCAGTA.<br>D)AGTCGA.<br>E)none
Q56: A solution that has a pH of
Q86: A sugar solution is an example of