Multiple Choice
Examine the activity list given below to answer this question: Which element is most easily oxidized,Mg ,Cu,or K ?
Activity Series = 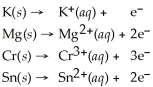 Cu(s) →
Cu(s) → 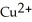 (aq) + 2 e-
(aq) + 2 e-
Definitions:
Related Questions
Q13: The oxidizing agent is the substance being
Q16: What molarity should the stock solution be
Q30: What types of forces exist between I<sub>2</sub>
Q41: How many kJ of heat are needed
Q46: A reversible reaction is one that can
Q72: What is the oxidation state of the
Q77: From the activity list included in this
Q111: Reduction typically involves:<br>A)the loss of electrons.<br>B)the gain
Q113: A positron particle comes from the decay
Q116: The effect of a catalyst is to:<br>A)increase