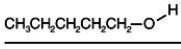
Consider the boiling points of the following compounds and their solubilities in room-temperature water.Why does the solubilities in water go down as the boiling points of these alcohols go up. 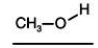

Definitions:
Net Operating Income
A measure of a company’s profitability, calculated as revenues less operating expenses excluding taxes and interest.
Traceable Fixed Expense
A fixed cost that can be directly linked to a specific segment of the business and would be eliminated if the segment ceased operations.
Percentage Change
A calculation that shows how much a quantity has increased or decreased in percentage terms over a period.
Traceable Fixed Expense
Fixed costs that can be directly associated with a specific product, department, or segment of a business.
Q1: Chemical equations need to be balanced
Q23: Which of the following molecules contains an
Q33: The element silicon has a melting point
Q55: If an alcoholic beverage has a proof
Q65: What do members of the Chemical Manufacturers
Q66: How would you describe the volume of
Q92: How does connecting a metal like iron
Q121: Describe what usually happens to a hot
Q121: An acid and a base react to
Q162: Water is 88.88 percent oxygen by