The boiling point of 1,4-butanediol is 230°C.Would you expect this compound to be soluble or insoluble in room-temperature water? 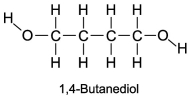
Definitions:
St. Louis Rams
A professional American football team that was based in St. Louis, Missouri, before relocating.
Bundling
The practice of selling several products or services together as a single combined unit.
Two-part Tariff
A two-part tariff is a pricing strategy that includes a fixed fee plus a variable charge based on the quantity of goods or services consumed.
Up-front Fee
An up-front fee is a charge or payment required before the receipt of a service or product, typically used to cover initial costs.
Q16: How is most of the energy required
Q18: Which two of these four structures are
Q20: Classify the following changes as physical or
Q47: Given the following diagram,describe what happens electronically
Q80: Since some of the compounds that are
Q87: A student is told to use 20.0
Q96: Why do the natural reactions involving ozone
Q100: The yeast in bread dough feeds on
Q105: According to the following reaction,which molecule is
Q164: What is the main characteristic of a