Dipole-induced dipole forces of attraction exist between water and gasoline,and yet these two substances do not mix because water has such a strong attraction for itself.Which of the following compounds might best help to make these two substances mix into a single liquid phase? 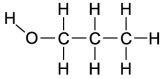
Definitions:
Socially Complex
Describes situations or constructs that are characterized by intricate interpersonal relationships and dynamics, often affecting group interactions and outcomes.
Unique History
A distinct and unrepeatable sequence of past events or experiences particular to an individual, organization, or place.
IO Perspective
Stands for Industrial Organization Perspective, focusing on the behaviors, structures, and policies affecting industries, including competition and regulation.
Producer Surplus
The difference between what producers are willing to accept for a good versus what they actually receive.
Q10: Based on atomic size,which would you expect
Q15: Just after an alpha particle leaves the
Q16: What is the name of the following
Q26: If you have two molecules of TiO<sub>2,</sub>
Q34: Is aging primarily an example of a
Q40: If you have one molecule of TiO<sub>2,</sub>
Q48: How is it possible for a neutral
Q51: What makes a semipermeable membrane selective for
Q90: Fatty acid molecules can also align to
Q126: What happens to the pH of an