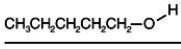
Consider the boiling points of the following compounds and their solubilities in room-temperature water.Why does the solubilities in water go down as the boiling points of these alcohols go up. 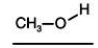

Definitions:
Social Learning Theory
A theory that suggests people learn new behaviors and norms through observation, imitation, and modeling from others in their environment.
Gender Differences
Variations in characteristics, behaviors, physical, psychological, and social traits typically associated with being male or female.
Mating Preferences
The criteria or qualities an individual looks for in a partner when choosing a mate.
Evolutionary Perspective
A theoretical approach that explains various aspects of human nature as a result of evolutionary processes that have shaped the survival and reproductive success of our ancestors.
Q10: If a baseball were scaled up to
Q16: Balance the following equation. _ NO →
Q22: What do the components of a conceptual
Q61: Which of the following elements is in
Q66: How would you describe the volume of
Q70: Which of the following elements are in
Q74: A certain radioactive element has a half-life
Q80: Which of the following might be considered
Q99: Which of the following statements describes a
Q99: An element is best described as a