The boiling point of 1,4-butanediol is 230°C.Would you expect this compound to be soluble or insoluble in room-temperature water? 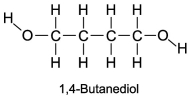
Definitions:
Expatriate Manager
A manager who is working in a country other than their own, often sent by their employer to manage an overseas office.
Agency Problems
Agency problems occur when there’s a conflict of interest between the principals (such as shareholders) and agents (such as company executives), leading to potential inefficiency or misuse of resources.
Specialized Knowledge
Expertise or skills in a specific field or subject area, typically acquired through education or experience.
Host-Country Nationals
Employees who are citizens of the country in which a multinational corporation's subsidiary operates, working in their own country for that subsidiary.
Q7: If the relative mass of a pingpong
Q28: Which of the following ethers has the
Q35: What is the main tenet of Plank's
Q36: Which of the following does not describe
Q50: Fish don't live very long in
Q66: Which molecule is most polar?<br>A)S=C=S<br>B)O=C=O<br>C)O=C=S<br>D)These all have
Q86: Which of the following statements best describes
Q101: How many moles of water,
Q120: Light will not pass through a pair
Q120: The reactants shown schematically below represent