Multiple Choice
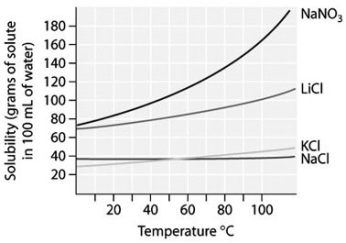
-At 10°C,which is more concentrated-a saturated solution of sodium nitrate,NaN ,or a saturated solution of sodium chloride? (See figure shown above.)
Comprehend the implications of price discrimination in service industries and why it is more prevalent there.
Understand regulatory approaches to monopolies and the concept of socially optimal pricing.
Evaluate the ethical considerations and consumer perceptions related to price discrimination practices.
Understand the concepts of socially optimal pricing and fair-return pricing in the context of regulating monopolies.
Definitions:
Related Questions
Q11: Does the average distance that a neutron
Q34: Is aging primarily an example of a
Q38: In a battery,the following two oxidation-reduction reactions
Q40: Why is the formation of iron
Q46: Which of the above substances would have
Q83: Which of the following statements is untrue
Q96: How can a hydrogen atom,which has only
Q103: What happens if you were to place
Q120: Which of the following types of radiation
Q173: In the above diagram,which atom is oxidized,the