Multiple Choice
Assuming a coordination complex is formed with Fe2+ and 1,10-phenanthroline (shown below) , which of the following statements is true 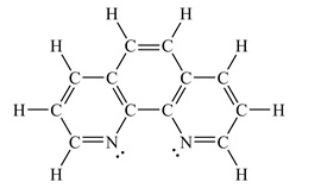
Definitions:
Related Questions
Q1: Protactinium-234 has a half-life of 1 minute.
Q18: Ozone (O<sub>3</sub>) and oxygen gas (O<sub>2</sub>) are
Q23: In Figure 1.3 A, B and C,
Q23: Which of the following choices is/are covalent
Q27: The following reaction is used to
Q28: The following balanced equation shows what
Q63: How much energy (kJ) is released
Q74: The best name for the complex shown
Q89: If there is an expectation that the
Q165: The cross price elasticity for computer peripherals