The isomerization of cyclopropane to propene follows first-order kinetics. 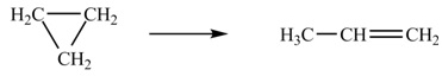 At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene
At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene
Definitions:
Social Loafing
The phenomenon where individuals exert less effort when working in groups than when working alone.
Social Loafing
The phenomenon where individuals exert less effort to achieve a goal when they work in a group than when they work alone.
Superordinate Goal
A shared goal that cannot be achieved by any one group or individual alone and requires cooperative effort.
Alcohol Abuse
The excessive or inappropriate consumption of alcohol leading to negative effects on an individual's health, social life, and responsibilities.
Q7: In which one of the following molecules
Q74: Given the following K<sub>a</sub> values, which
Q95: A certain reaction A <span
Q98: The gas phase exists at the point
Q103: Which one of these statements about strong
Q105: For which of the following species are
Q108: The solubility of silver bromide can
Q122: According to VSEPR theory, which one of
Q134: What is the hybridization on the central
Q142: Starting with 0.750L of a buffer solution