Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm 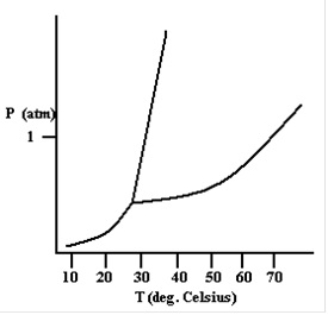
Definitions:
Statement of Cash Flows
A financial report that describes the sources of a company's cash and how it was spent over a specific time period.
Financing Activities
Transactions and events where cash is raised from or repaid to investors, such as issuing equity or debt.
Preferred Stock
A class of ownership in a corporation that has a higher claim on assets and earnings than common stock, usually with predetermined dividend payments.
Statement of Cash Flows
A financial report that shows how changes in balance sheet accounts and income affect cash and cash equivalents.
Q31: A bonding molecular orbital is of lower
Q34: The N - N - H
Q44: Which pair of elements from different groups
Q58: A sp<sup>2</sup> hybridized central atom has
Q64: Use bond energies to estimate the enthalpy
Q96: Which one of the following ions has
Q101: Calculate the amount of heat needed
Q112: The reaction A(g) + 2B(g)
Q114: At a particular temperature the first-order
Q116: At 400ºC, K<sub>c</sub> = 64 for the