Carbonic acid, H2CO3, is a weak acid that contributes to the taste and produces the carbon dioxide bubbles in all carbonated beverages. How many valence electrons are used to show the Lewis structure for H2CO3 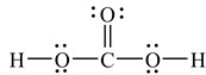
Definitions:
Successive Years
Consecutive years following one immediately after the other.
Missing Interest Rate
An interest rate that is not specified or known in a financial scenario, which may need to be calculated based on other given information.
Missing Interest Rate
The unknown rate of interest in a financial calculation that needs to be determined.
Missing Interest Rate
The interest rate that is not stated or known in a financial problem, which may need to be calculated.
Q8: List all of the bond angles
Q10: Which of the elements listed below would
Q12: The ground-state electron configuration for an atom
Q21: Which, if any, is the ground-state electron
Q21: Which of the following correctly lists species
Q24: All intermolecular forces must be overcome in
Q35: If the pressure of a gas sample
Q61: If a hydrogen atom and a helium
Q92: The normal boiling point of bromine
Q123: The following reaction correctly shows the