The structure below depicts the correct Lewis structure for Dichloromethane, CH2Cl2 (an important solvent in synthetic chemistry). 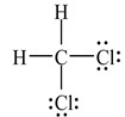
Definitions:
Flexor Carpi Ulnaris
A muscle of the forearm that flexes and adducts the hand.
Anatomical Description
The process of detailing the structure and location of organs and systems within an organism.
Shoulder
A complex joint connecting the arm to the torso, encompassing several bones, muscles, and tendons.
Wrist
The joint connecting the forearm to the hand, encompassing eight small bones known as the carpal bones.
Q8: Arrange the following in order of increasing
Q18: The bond angles in ICl<sub>2</sub><sup>-</sup> are
Q25: What is the osmotic pressure of
Q37: Which one of the following sets of
Q94: Which ion is isoelectronic with Ar<br>A) Fe<sup>2+</sup><br>B)
Q99: The Lewis dot symbol for the
Q116: A 100. mL sample of 0.200
Q118: Predict the geometry around the central atom
Q121: What is the pressure of the sample
Q123: The quantum numbers, n = 4, l