Carbonic acid, H2CO3, is a weak acid that contributes to the taste and produces the carbon dioxide bubbles in all carbonated beverages. How many valence electrons are used to show the Lewis structure for H2CO3 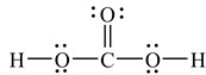
Definitions:
Premium
An amount paid for an insurance policy, or the amount to acquire a better service or product version.
Pillsbury Bake-Off
A cooking contest, initiated in 1949, where contestants from across the United States compete by preparing original recipes with predefined products from the Pillsbury Company.
Product Usage
Product usage concerns how consumers interact with and utilize a product, including frequency, purpose, and manner of use.
Contest
A competition where individuals or groups compete for a prize or recognition.
Q2: Which of the following is the electron
Q30: Which of the following is the general
Q49: The second line of the Balmer series
Q49: Calculate the density, in g/L, of chlorine
Q74: Given the specific heat for aluminum
Q77: The heat of neutralization of HCl
Q79: What is the energy in joules of
Q122: The solubility of a solid always increases
Q145: According to the VSEPR theory, the geometry
Q147: The most space efficient arrangement of spheres