Shown here is a Lewis structure for SO3 that expands the octet to minimize formal charges. The formal charge on the sulfur atom is zero. 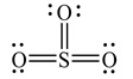
Definitions:
Molecular Orbital
A region in a molecule where electrons are most likely to be found; it represents the wave-like behavior of electrons in molecules.
Atomic Orbitals
Mathematical expressions that characterize the wave-like patterns of electrons within an atom.
Orbitals
Mathematical functions that describe the wave-like behavior of electrons in atoms, important for understanding the chemical bonding of atoms.
Mathematical Description
The use of mathematical symbols and formulas to represent and solve real-world problems or theoretical concepts.
Q4: Given the following <span class="ql-formula"
Q7: If 2Mg(s) + O<sub>2</sub>(g) <span
Q43: Use the bond enthalpy data given
Q49: The second line of the Balmer series
Q64: Calculate the density of Br<sub>2</sub>(g) at
Q71: Based on the phase diagram shown below,
Q84: Which one of the following elements is
Q99: The vapor pressure of water at
Q117: A possible set of quantum numbers to
Q142: Since zirconium is a metal, ZrO<sub>2</sub> is