Determine the mass of BaSO4 that is produced by the reaction of 45.0 mL of 0.155 M H2SO4 and 60.0 mL of 0.125 M BaCl2. Assume that BaSO4 is totally insoluble. 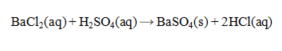
Definitions:
Independent Variable
A variable that is manipulated in an experiment to see if it affects the outcome or dependent variable.
Dependent Variable
In scientific research, the variable that is tested and measured to determine if it is affected by changes in the independent variable.
Self-critical
A tendency to evaluate oneself harshly, focusing on one’s flaws and shortcomings.
European
Pertaining to a person from, or associated with, the continent of Europe.
Q4: Chemical sedimentary rocks are those precipitated from
Q13: Sources of heat in the early earth
Q15: The compositions of the planets<br>A) Are all
Q20: Which process involves a decrease of entropy
Q24: Anions are negatively charged and cations are
Q26: Which of the following indicator(s) would be
Q43: Expansive clays<br>A) Expand when wet, shrink when
Q47: The process of dissolving is favored if
Q53: Which element has the largest atomic radius?<br>A)
Q61: Which statements about resonance are true?<br>I. Resonance