Ammonia and sulfuric acid react according to the equation given below. How many milliliters of 0.110 M sulfuric acid are required to exactly neutralize 25.0 mL of 0.0840 M NH3 solution? 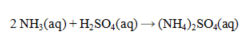
Definitions:
Desired Results
The specific outcomes or objectives that an individual, group, or organization aims to achieve, serving as targets for planning and performance evaluation.
Formal Training And Education
Structured programs designed to develop skills and knowledge necessary for specific jobs or careers through classes, workshops, and practical exercises.
Honeywell
A multinational conglomerate company that produces a variety of commercial and consumer products, engineering services, and aerospace systems.
Managing
The process of planning, organizing, leading, and controlling resources to achieve organizational goals effectively and efficiently.
Q2: Stoichiometric coefficients found in a balanced equation
Q2: Phenolphthalein is an acid-base indicator that is
Q19: An island arc comprising of volcanic islands
Q22: Exhibit 16-1 The following question(s) refer to
Q24: Does the metric system of measurement have
Q35: Which molecule does not contain a multiple
Q42: The process by which sediments are converted
Q45: Give an example of an Alkaline Earth
Q45: For the dissociation of a very weak
Q49: Which statement about bond energies is false?<br>A)