Oxalic acid, H2C2O4, reacts with Y(NO3) 3 as shown by the equation below. What weight of yttrium oxalate is produced from 50.0 mL of 0.265 M Y(NO3) 3 and excess oxalic acid? Assume that all of the yttrium oxalate is insoluble. 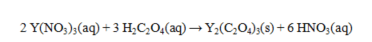
Definitions:
Non-volume Factors
Elements that affect a business’s operations and cost structures that are not related to the volume of goods or services produced, such as changes in technology or regulatory environments.
Non-manufacturing Costs
Expenses not directly tied to the production process, such as selling, general, and administrative expenses.
Non-manufacturing Overhead
Expenses incurred by a company that are not directly related to the production process, such as administrative and marketing expenses.
Traditional Approach
A conventional method often based on long-established practices or techniques.
Q7: Assume that only two isotopes of argon,
Q7: Which of these does not correspond to
Q9: London forces exist<br>A) for all molecules.<br>B) only
Q13: Label the hybridization at C#1, C#2, C#3,
Q31: Lack of habitable land on earth is
Q36: Which of the following statements about two
Q41: Give the formula for the ionic compound
Q48: Find the correct combination of protons and
Q51: An organic compound contains 60.0% carbon, 4.44%
Q53: The molecular formula for copper (II) oxide