Determine the mass of BaSO4 that is produced by the reaction of 45.0 mL of 0.155 M H2SO4 and 60.0 mL of 0.125 M BaCl2. Assume that BaSO4 is totally insoluble. 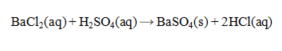
Definitions:
Joint Return
Joint Return is a tax filing status that allows married couples to combine their income, exemptions, deductions, and credits on a single tax return.
AGI
A computation of your earnings from your overall income, Adjusted Gross Income is employed to identify the amount of your earnings that is subject to tax.
Child And Dependent Care Credit
A tax credit offered to taxpayers for expenses related to the care of children under 13 or disabled dependents, enabling the taxpayer to work or look for work.
Dependent Child
A child who relies on another person for more than half of their support and qualifies for the potential of tax benefits for the supporting individual.
Q6: Write the correct Lewis dot structures for
Q19: An island arc comprising of volcanic islands
Q20: Classify the following reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Classify
Q20: The Anatolian Fault Zone in Turkey illustrates
Q22: For which molecule will the electron-pair geometry
Q22: A wine sample had a pH of
Q25: Of the following rocks,one that is metamorphic<br>A)
Q35: Calculate the value of DS<sup> <span
Q44: There are _ milligrams in a kilogram.<br>A)
Q63: Which statement about molecular orbital theory is