Ammonia and sulfuric acid react according to the equation given below. How many milliliters of 0.110 M sulfuric acid are required to exactly neutralize 25.0 mL of 0.0840 M NH3 solution? 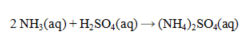
Definitions:
Individual's Feelings
Personal emotions or affective states that are unique to an individual, reflecting their personal reactions or sentiments.
Perceived Inequity
The feeling or perception that one's rewards or outcomes are not fair in comparison to others' rewards or outcomes, leading to feelings of injustice.
Quantity
A measurable amount or the total number of units of a product, material, or substance.
Quality Of Work
The degree to which a task or project is executed with excellence, precision, and effectiveness, meeting or exceeding standards.
Q6: Write the correct Lewis dot structures for
Q8: The magnitude of the earthquake is dependent
Q11: How many electrons will be in the
Q24: The Appalachian Mountains of eastern North America
Q42: Which of the following statements concerning the
Q43: Which element has four electrons in its
Q46: A reaction is product-favored when<br>A) K<sup>
Q47: A solution is made by dissolving 60.0
Q50: Use the data given to calculate
Q59: Radioactive atoms emit alpha and _ particles.