Multiple Choice
How many grams of Fe2O3 are formed by the complete reaction of 6.75 moles of iron? 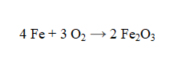
Definitions:
Matching Principle
An accounting rule that dictates expenses should be recorded in the same period as the revenues they helped to generate.
Related Questions
Q7: A subgroup of silicates that includes minerals
Q12: Samples of which substance experience only London
Q14: _ containing compounds are known as organic
Q14: Use the VSEPR model to predict the
Q18: The driving force for osmosis is<br>A) temperature.<br>B)
Q20: If cadmium metal and the Fe(III) ion
Q22: For which molecule will the electron-pair geometry
Q30: Which of the following is not a
Q34: Which statement is not correct?<br>A) an electrochemical
Q39: Which statement about light is true?<br>A) Light