In the reaction given below, how many grams of C14H9Cl5 will be produced by the reaction of 25.0 g of each of the starting materials? 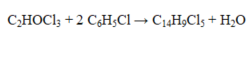
Definitions:
Upstream Commerce
The activities involved in the sourcing and procurement processes before goods reach the retailer or final consumer.
Water Transportation
A method of transporting goods and people using waterways, including oceans, rivers, lakes, and canals, utilizing various types of vessels.
Road Transportation
The movement of goods and people from one place to another over roads, using vehicles such as cars, trucks, and buses.
War of 1812
The War of 1812 was a military conflict fought between the United States and the British Empire, primarily over maritime rights and territorial expansion, strengthening U.S. nationalism and leading to a period of peaceful relations.
Q8: Which sample contains the same number of
Q16: Refer to the following values of standard
Q37: Two substances, A and B, have
Q46: Which compound will not dissolve in water
Q47: Plate tectonics are unrelated to the rock
Q50: Which group contains only solutes that would
Q50: Determine the incorrect relationship given below.<br>A) 1
Q55: Assume all hydrocarbons given are linear. Which
Q57: In Rutherford's gold foil experiment:<br>A) the alpha
Q57: Methylamine, CH<sub>3</sub>NH<sub>2</sub>, acts as a weak base