In the reaction given below, how many grams of water are consumed if 4.0 g of hydrogen gas and 32.0 g of oxygen gas are produced? 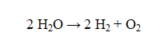
Definitions:
Manipulating
The process of altering variables in an experimental setting to determine their effects on dependent variables.
Variables
Elements, factors, or conditions that can change and affect the outcomes of experiments and studies in various fields, especially in research and statistics.
Dependent Variable
The variable being tested and measured in an experiment, which is expected to change as a result of manipulations of the independent variable.
Industrial Designer
A professional who develops concepts and designs for manufactured products, focusing on aesthetics, functionality, and usability.
Q4: Which of the following refers to a
Q9: The internal regular arrangement of ions or
Q22: In which of the following areas is
Q23: Write the net ionic equation for the
Q34: Which acid-base titration would yield a titration
Q34: Five coins are tossed. Which combination of
Q40: In a buffer solution, if [A<sup>-</sup>] =
Q47: The relationship between Gibbs free energy
Q58: The seismic-risk map of the United States
Q63: Which statement about molecular orbital theory is