How many moles of H2O are formed from the complete combustion of 45.0 g of methanol, CH3OH? 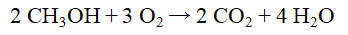
Definitions:
Imports
Goods and services that are brought into a country from abroad for sale.
Decreases
A reduction or decline in value, amount, or level.
Constant Dollar
A term used to describe a dollar value that has been adjusted for inflation to reflect purchasing power at a specific point in time.
Real GDP
Gross Domestic Product adjusted for inflation, measuring the value of all goods and services produced by an economy in real terms.
Q7: Determine the energy of a photon that
Q8: In terms of acid strength, which acid
Q9: Consider a 0.50 M solution of HNO<sub>2</sub>,
Q15: The first ionization energy is the amount
Q22: What is the molar heat capacity of
Q28: Consider the cell reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg"
Q40: The standard enthalpies of formation for several
Q54: Which ionic compound is expected to have
Q56: A solution with solute concentration equal to
Q58: One water molecule can donate a proton