In the reaction given below, how many grams of C14H9Cl5 will be produced by the reaction of 25.0 g of each of the starting materials? 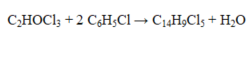
Definitions:
Sales Returns
Goods returned by customers to the seller for refund or exchange due to defects, dissatisfaction, or other reasons.
Cash Discounts
Reductions in the amount owed by a customer if payment is made within a specified period, incentivizing early payment.
Direct Write-off Method
A method for managing uncollectible accounts where specific debts are directly written off against income at the time they are deemed unrecoverable.
Cash Realizable Value
The amount of money that can be received from assets or receivables through normal collection processes.
Q17: At constant T and P, in which
Q22: What is the molar heat capacity of
Q25: Which compound is incorrectly named?<br>A) Ni(NO<sub>3</sub>)<sub>2</sub> nickel(II)
Q29: Ions that contain atoms of more than
Q31: At constant T and P, in which
Q35: When conducting a titration of an acid
Q36: The half-life of radon-222 is 2.8 days.
Q41: What is the oxidation number of P
Q44: The particle grain size conglomerate is greater
Q61: An acidic solution is diluted until [H<sub>3</sub>O<sup>+</sup>]